![]() Based on Orphanbiotec’s special collaboration with expert partners such as the Foundation Orphanhealthcare and other R&D partners, it is possible to identify active substances very early and cost-effectively, and translate them into new pharmaceutical innovation. We also make use of the experiences of those concerned and the available open databases of our research partners.
Based on Orphanbiotec’s special collaboration with expert partners such as the Foundation Orphanhealthcare and other R&D partners, it is possible to identify active substances very early and cost-effectively, and translate them into new pharmaceutical innovation. We also make use of the experiences of those concerned and the available open databases of our research partners.
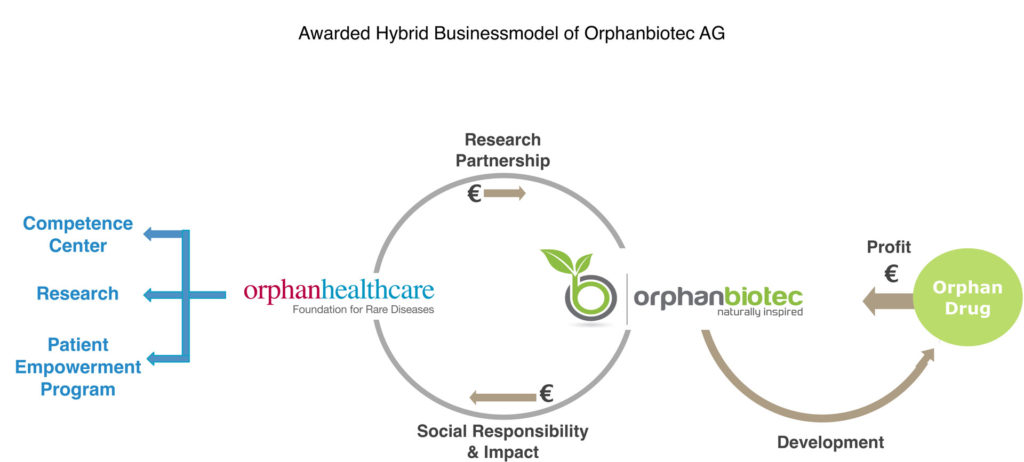
Pre-financing by the research foundation in the pre-clinical phase (proof of concept) makes it possible to explore the critical period of development and adopt successful candidates into the clinical phase. They are pre-developed for the best form of administration (oral, intravenous, subcutaneous, etc.) based on the application to guarantee a high efficacy while minimizing side effects. Orphanbiotec AG then develops these candidates further together with specialized partners from the contract research organizations (see figure), and they are then produced and sold by our partners and patented and approved by Orphanbiotec. Limited investment, short development time and shared risk are the key factors of success.